Classification of Edible Salt Products by Tandem LA-ICP-MS and LIBS Analysis
LIBS and LA-ICP-MS have been studied and developed extensively for sample classification and discrimination technology for forensic, geological, gemological, and environmental applications. Recent advances in LA instrumentation now makes it possible to obtain LIBS and LA-ICP-MS data simultaneously, allowing analysts to obtain more complete chemical fingerprinting information about a sample.
The blog piece below highlights the research work of Dr. Yonghoon Lee at Mokpo National University to apply Tandem LA-ICP-MS and LIBS analysis for discrimination of salts from different geographical origins. More detailed technical discussion of this research was published in Spectrochimica Acta Part B 118 (2016) 102-111.
By Dr. Yonghoon Lee, Department of Chemistry, Mokpo National University, Republic of Korea
An average person is thought to consume a few to 10 g of salt per day. A wide range of edible salt products from different countries is available in local food markets. However, the price of salt products may vary significantly from cheap table salt to more expensive mineral-rich sea salts and pink/black rock salts. The large difference in salt price often encourages illegal circulation of fake salt products. Therefore, it is desirable to establish reliable fingerprints of salt products and measurement techniques to test these products.
Chemical composition can be utilized as the unique fingerprint for different types of salts. Salt is a mixture of various ionic compounds in the sodium chloride (NaCl) matrix. The concentration of magnesium (Mg), calcium (Ca), and potassium (K), ranging from several hundred ppm to a few %, depends on the production method and surrounding environment of saltpans or salt mines. These are the main metallic impurities in salts.

Laser Induced Breakdown Spectroscopy (LIBS) is a rapid and versatile elemental analysis technique. The optical emission from the laser induced plasma conveys rich information about the chemical composition of samples. LIBS is particularly effective for the analysis of light metallic elements such as Na, K, Mg, and Ca. Thus, LIBS spectra can be chemical fingerprints of salts, indicating their geographical origins and production methods. Rich chemical information in the LIBS spectra can be effectively extracted and utilized for developing classification models involving PCA analysis.
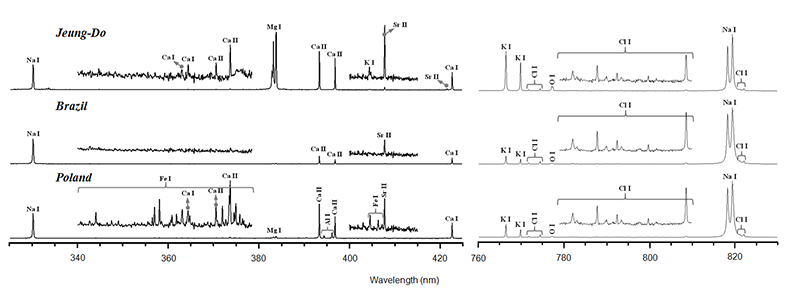
Accuracy in salt classification can be improved further by combining orthogonal chemical information with respect to LIBS data. One of the elemental analyses that can be performed simultaneously with LIBS is Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS). LIBS and LA-ICP-MS share the same solid sampling and sample excitation source, laser ablation.
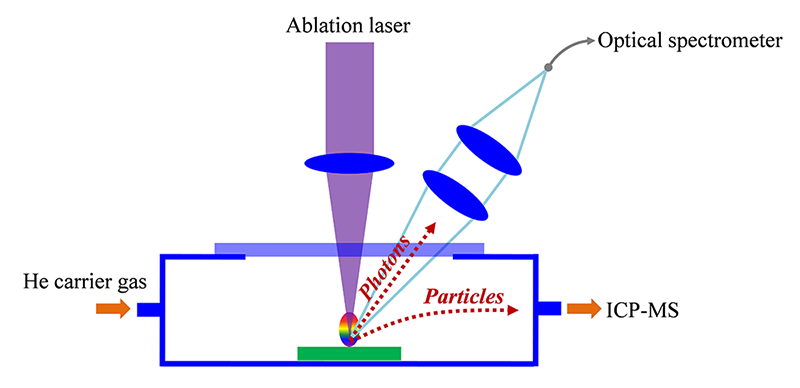
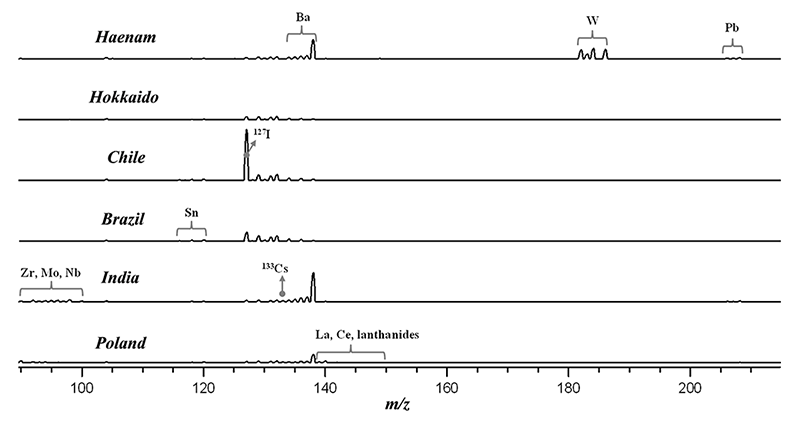
From the laser induced plasma, LIBS detects photons and LA-ICP-MS samples particles for ICP-MS. LIBS analyzes light metallic elements (K, Mg, Ca, etc.) contained in salts with relatively high concentrations (ppm to %). LA-ICP-MS can be applied for other elements in the periodic table such as non-metallic elements and heavy metals present especially at lower concentration.
The two different data from LIBS (optical emission spectra) and LA-ICP-MS (mass spectra) have been found to be independent from each other. LIBS spectra provide chemical information mainly about K, Mg, and Ca whose concentration varies by different production methods and interaction of rock salts with underground fresh water. The heavy metal LA-ICP-MS mass spectra may represent the composition contribution from the environment surrounding saltpans or salt mines. Also, iodized salt products are easily discriminated by the 127I mass peak.
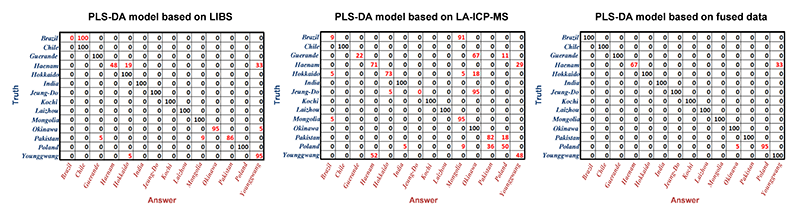
The ideal data set for developing a classification model is prepared by combining LIBS and LA-ICP-MS spectra with optimized weighting factors.
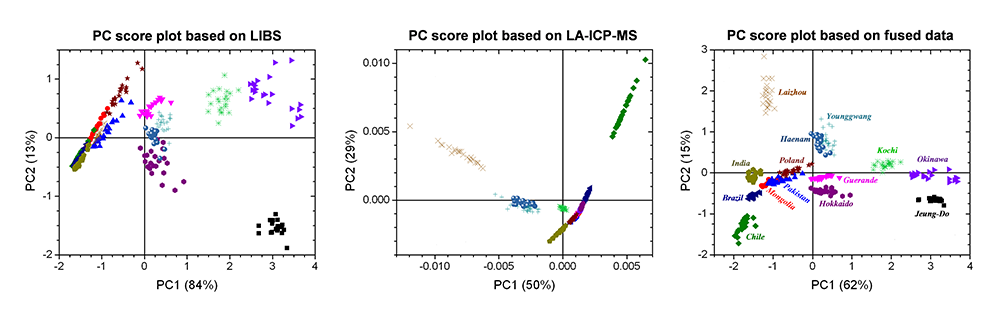
The evaluation of the PLS-DA model performance based on LIBS, LA-ICP-MS, and their fused data indicates that LIBS and LA-ICP-MS provide chemical information complimentary to each other.
Thus, the model based on the combined data from LIBS and LA-ICP-MS led to improved classification accuracy. R&D collaboration among Mokpo National University, Lawrence Berkeley National Laboratory, and Applied Spectra revealed that tandem LA-ICP-MS and LIBS analysis is effective in capturing more complete chemical fingerprints of salts for accurate classification (Y. Lee, et al., Multivariate Classification of Edible Salts: Simultaneous Laser-Induced Breakdown Spectroscopy and Laser-Ablation Inductively Coupled Plasma Mass Spectrometry Analysis, Spectrochimica Acta Part B 118 (2016) 102-111).
In recent years, LIBS, in combination with multivariate analysis, has proven itself as a reliable material classification technology. Classification of salts is one representative example of the expanding applications of LIBS. In the future, LIBS could be hyphenated with other spectroscopic techniques, such as LIBS-LA-ICP-MS, LIBS-Raman, LIBS-LIF, LIBS-IR, etc., for developing highly accurate material screening analytical methods.

