Direct Determination of Contaminants and Major and Minor Nutrients in Solid Fertilizers Using Laser Induced Breakdown Spectroscopy (LIBS)
With a growing world economy and population, the world today faces the challenge of supplying adequate staple crops for global human consumption. At the heart of this challenge is soil fertilization to ensure soil contains needed nutrients for the best crop yield without contamination hazardous to human health.
In the blog piece below, researchers at the Department of Chemistry (DQ), Center of Exact Science and Technology (CCET), Federal University of São Carlos (UFSCar) discuss how LIBS can be used to perform rapid chemical quality control of nutrients and toxic elements for fertilizer samples. More detailed technical discussion of this research was published in the Journal of Agricultural and Food Chemistry 2016, 64, 7890−7898.
By MSc. Daniel F. Andrade and Dr. Edenir Rodrigues Pereira-Filho, Federal University of São Carlos (UFSCar)
The formulation of fertilizer contains critical nutrients essential for plant growth. The right fertilization of soil enhances the chance for high crop yield and helps satisfy the growing demand of the world for staple crops. On the other hand, the fertilizer may contain potentially toxic elements from rocks, minerals, and other materials used for its production. Elemental analysis for quality control of the fertilizer before the application on the soil is crucial to verify the proper content of macro and micronutrients and level of toxic elements.

Due to its direct solid analysis capability, Laser Induced Breakdown Spectroscopy (LIBS) has been employed in a wide range of applications in recent times. Using a single laser pulse, it is possible to detect fingerprint-like LIBS spectra representing atomic and molecular species present in the fertilizer samples. Some of the major advantages of this technique are fast measurement speed and simultaneous detection of the elements with little to no sample preparation.

Direct fertilizer sample analysis was carried out using the J200 LIBS Instrument. Before the analysis, the samples were pressed to form pellets and the LIBS spectra were collected from these fertilizer pellets. The emission lines for B, Ca, Cd, Cr, Cu, Mg, Mn, Na, Pb, and Zn were monitored. Chemometric strategies were employed to optimize the instrument’s operational conditions and selection of analytes’ emission lines free of interference.
The concentration of the elements determined by LIBS ranged from 5 ppm (Cd) to 18% (Mg). This study revealed that Pb, a hazardous metallic element, was found in some tested samples at alarming concentration levels (from 0.05 to 1.1%). For some elements, such as Cu, Mg, Mn, Na and Zn, good regression models were obtained using more than one analytical emission line. Figure 1 shows the accuracy evaluation for Mn based on comparison between the ICP OES measured concentrations and LIBS predicted values.
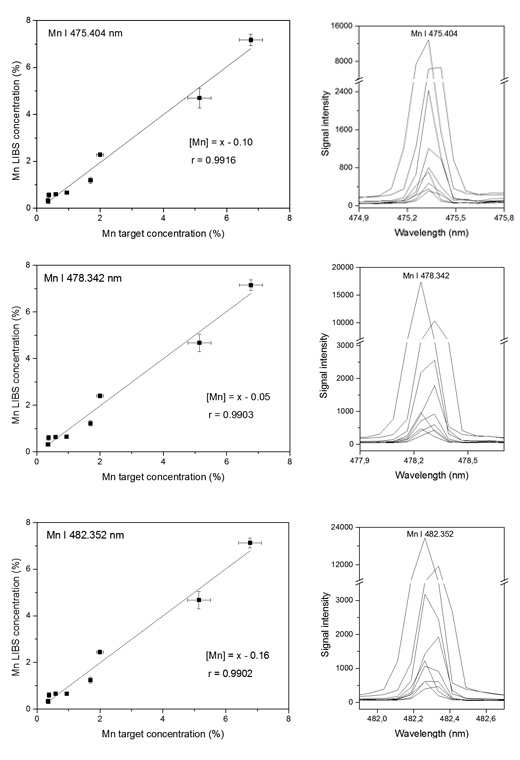
Adapted with permission from the Journal of Agricultural and Food Chemistry, 2016, 64, 7890-7898. Copyright 2016 American Chemical Society.

