Determination of Elemental Composition of Marcellus Shale Rocks by LIBS
By Drs. Jinesh Jain and Dustin McIntyre, National Energy Technology Laboratory
Exploration of shale oil has received tremendous interest in recent years as the discovery for an alternate oil and natural gas source intensifies. The elemental analysis for shale samples during the exploration stage is essential to assess cost of the extraction and the quality of oil and natural gas and control waste products. In the blog piece below, Drs. Jinesh Jain and Dustin McIntyre from NETL (National Energy Technology Laboratory) discusses using LIBS (Laser Induced Breakdown Spectroscopy) to perform rapid elemental analysis for shale samples. More detailed technical discussion of this research was published in Spectrochimica Acta Part B 122 (2016) 9–14.
By Drs. Jinesh Jain and Dustin McIntyre, National Energy Technology Laboratory
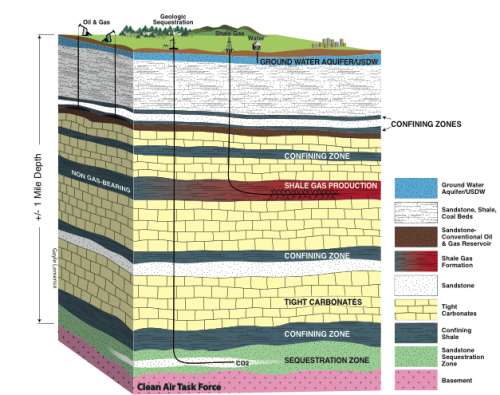
The organic-rich shale formations (i.e., Marcellus shale), storing significant amounts of natural gas, not only serve as a source for the gas but also form the reservoir. In addition, the depleted shale gas reservoirs have been proposed as candidates for geologic storage of CO2 and potential enhanced gas recovery (Figure 1).
Elemental analysis of shale rocks is of interest because, 1) both CO2 and natural gas (CH4) adsorption/desorption seem to be correlated with mineral composition of the rocks, 2) the rocks that contain higher amounts of carbon contents (organic material) have greater ability to generate natural gas and potentially a greater capacity of CO2 storage, and 3) environmental issues associated with shale retorting require substantial monitoring and control of waste products.
Utilization of LIBS for elemental analysis of shale rocks is advantageous because it enables a rapid in situ analysis with little or no sample preparation and can perform multi-element analysis including total carbon.
A horizontal drilling rig for natural gas in the Marcellus formation in eastern Lycoming County, Pennsylvania.
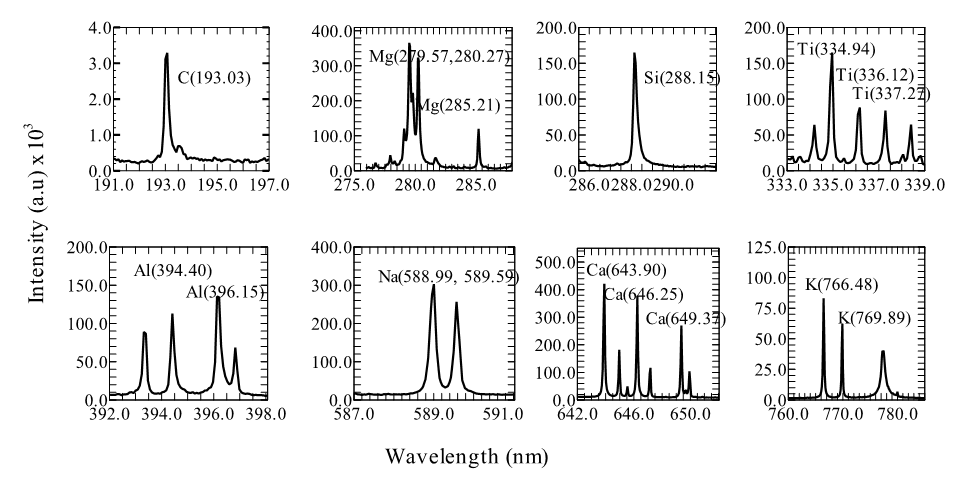
Measurements were performed on pressed pallets of Marcellus shale samples using J200 LIBS Instrument (EC model). The elements of interest (Al, Ca, K, Ti, Na, Si, and C) were spectrally resolved (Figure 2).
Fragments below exposure of fissile Marcellus black shale at Marcellus, N.Y.
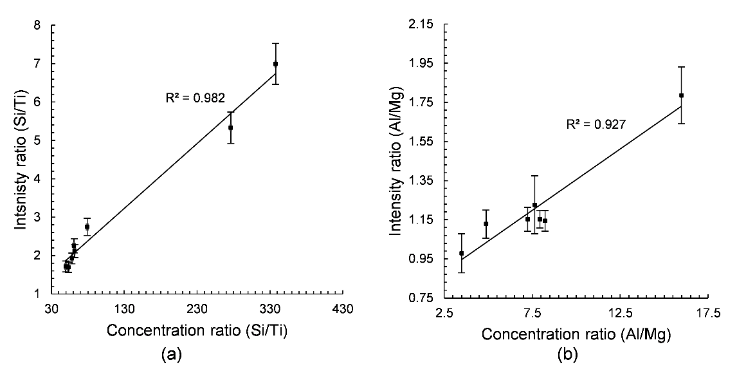
Figure 3 displays the calibration curves of C, Al, Mg, Ti, Ca and Si with outliers.
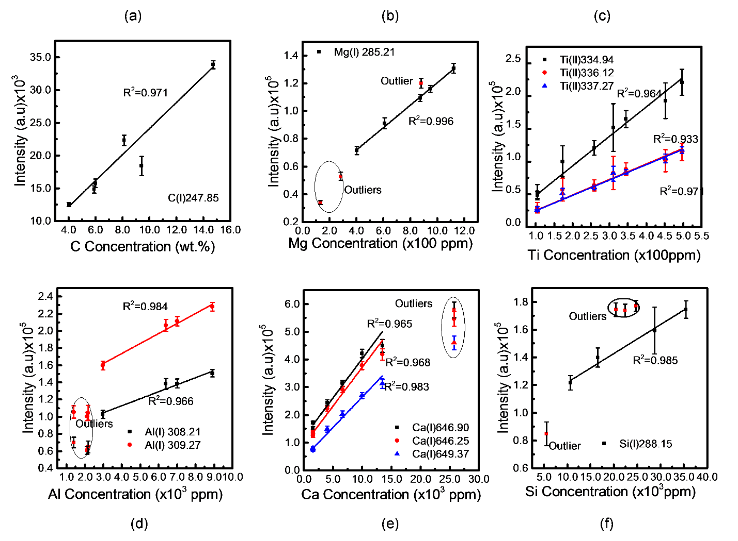
Figure 4 shows that the outliers are associated to the influence of other elements notably Mg and Ti. As for Ca, it is probably related to its high concentration. The limit of detections (LOD) obtained from the above calibration curves for Si, Al, Ti, Mg and Ca are 60.9, 33.0, 15.6, 4.2 and 0.03 ppm, respectively while it is 0.5 wt% for carbon. In conclusion, LIBS can provide a rapid analysis of shale samples and can potentially benefit depleted gas shale carbon storage research.